The “deletion” mouse was developed by GESC internal R&D with Mouse Genetics Core (MGC, link https://mgc.wustl.edu/ ) to improve efficiency in creating mouse models for tissue specific overexpression from the ROSA26 locus.
There are frequent requests to create mice by inserting into the ROSA26 safe harbor site a cassette of the following configuration to overexpress an open reading frame (ORF) only in Cre recombinase positive cells in the body:

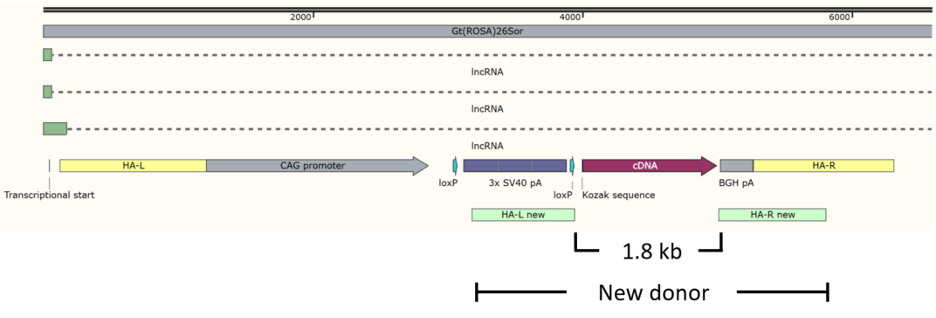
Except for the ORF to be expressed, the rest of the cassette remains the same in different models and yet is 3 kb long. The “deletion” mouse has CAG promoter, floxed 3x SV40 pA and BGH pA but no cDNA.
An example of the original donor design used previously
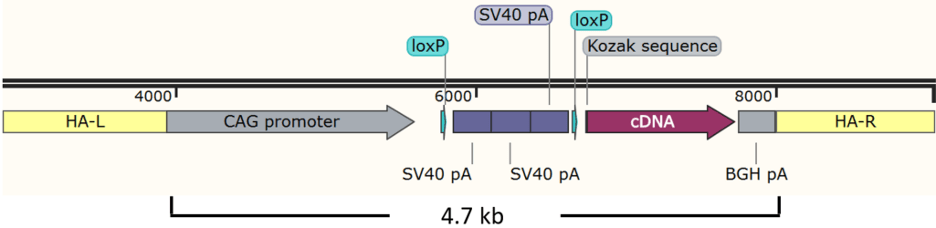
The total insert is 4.7 kb for a cDNA of 1.8 kb. Larger insertions tend to be less efficiently incorporated into the genome in zygotes, even with the assistance of CRISPR.
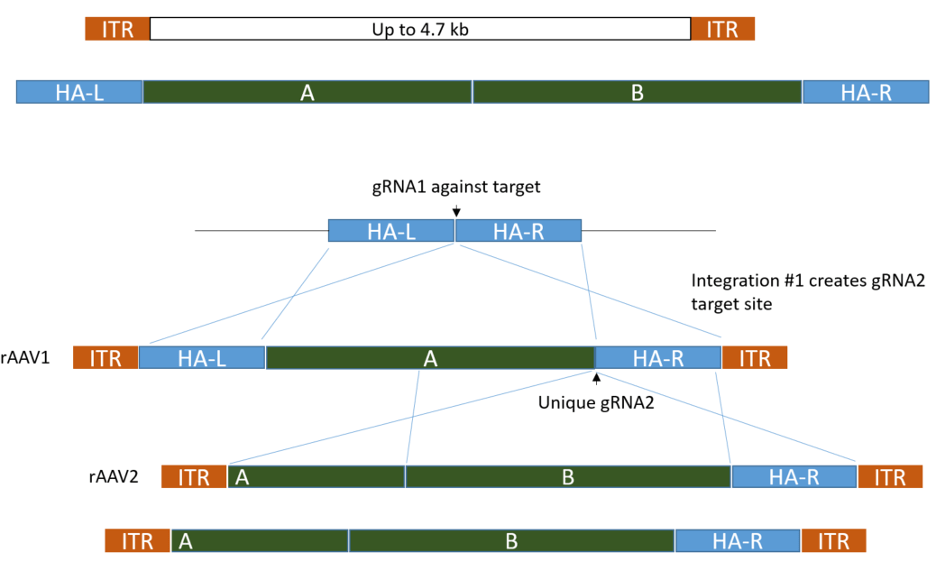
Insertion of a large cassette by TALENs or CRISPR![]()
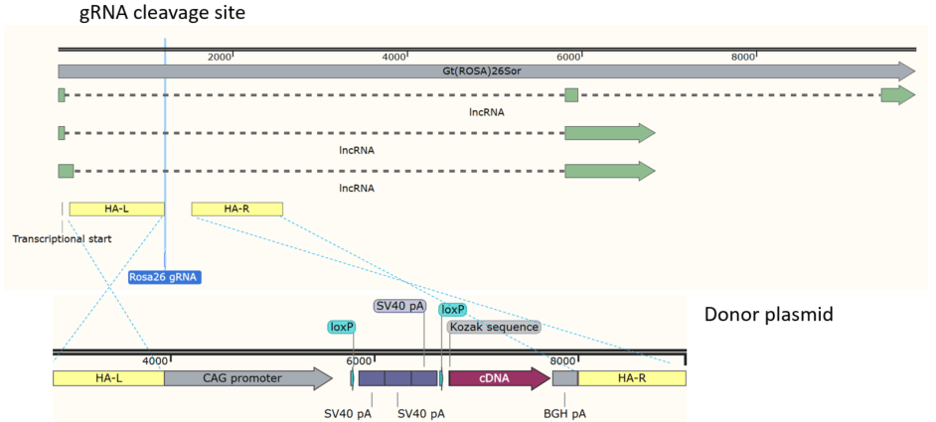
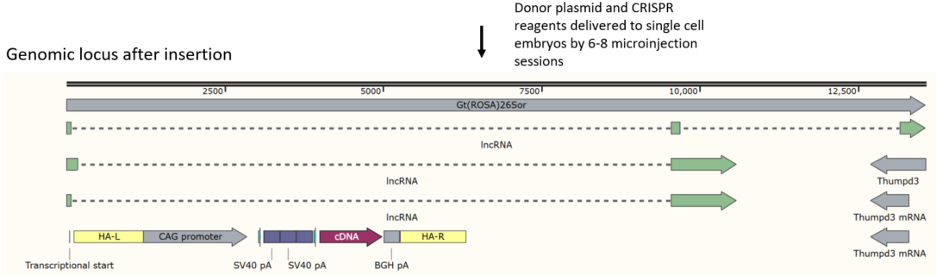
“Deletion” mouse created by removing cDNA from an existing model
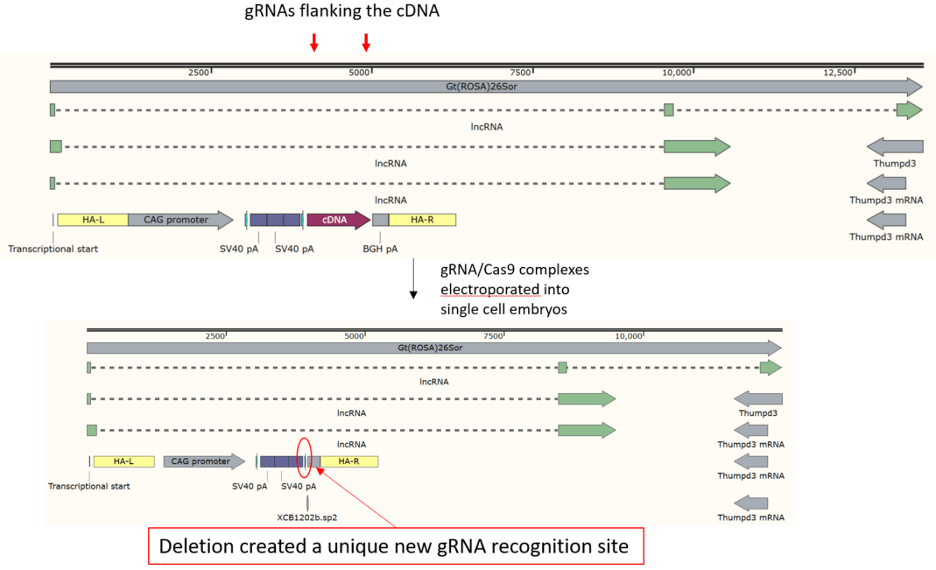
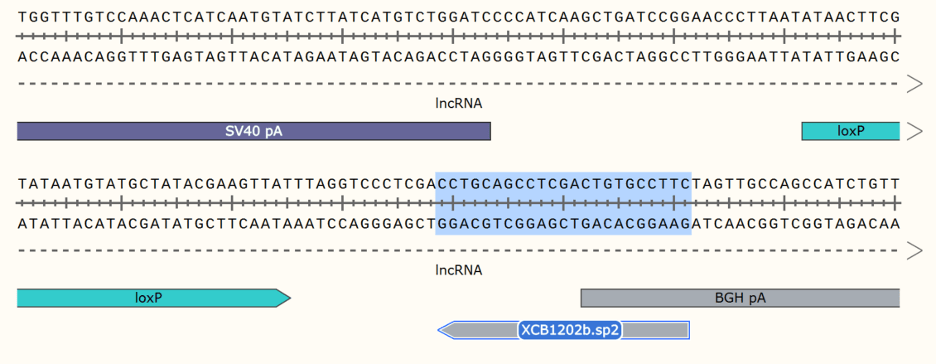
Unique, new gRNA site for inserting other ORFs between floxed stop and
polyA signal
Only coding sequences have to be inserted in the “Deletion” mouse
In conjunction of rAAV donors, creating a new model for conditional overexpression can be achieved by one or two electroporation sessions.
Large knockin by rAAV donor transduction vs. donor plasmid microinjection
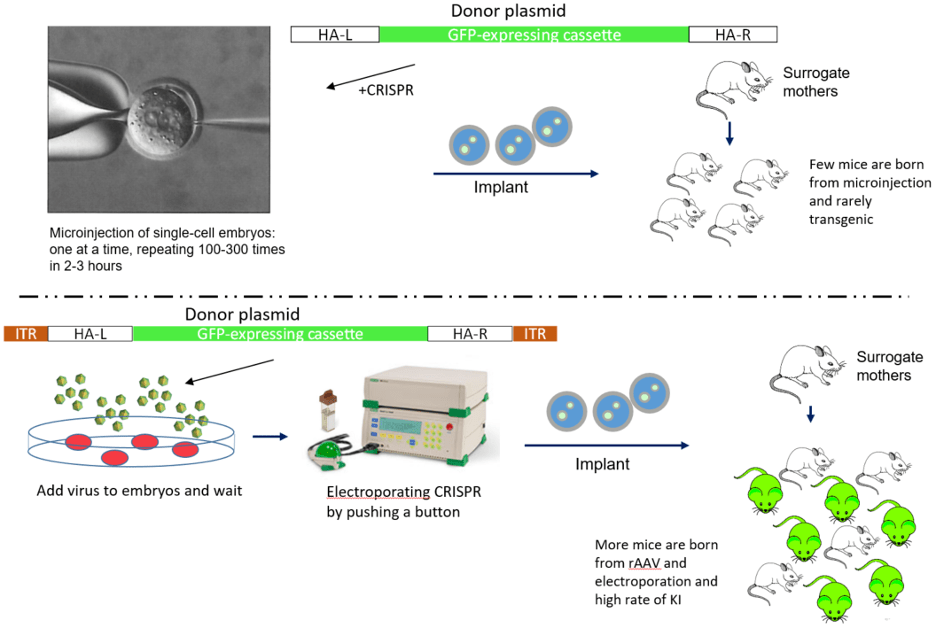
Even larger insertions can be delivered efficiently by using two rAAVs